Sommaire de la page
Thématique de recherche

Dr Isabelle Schalk, Dr Gaëtan Mislin
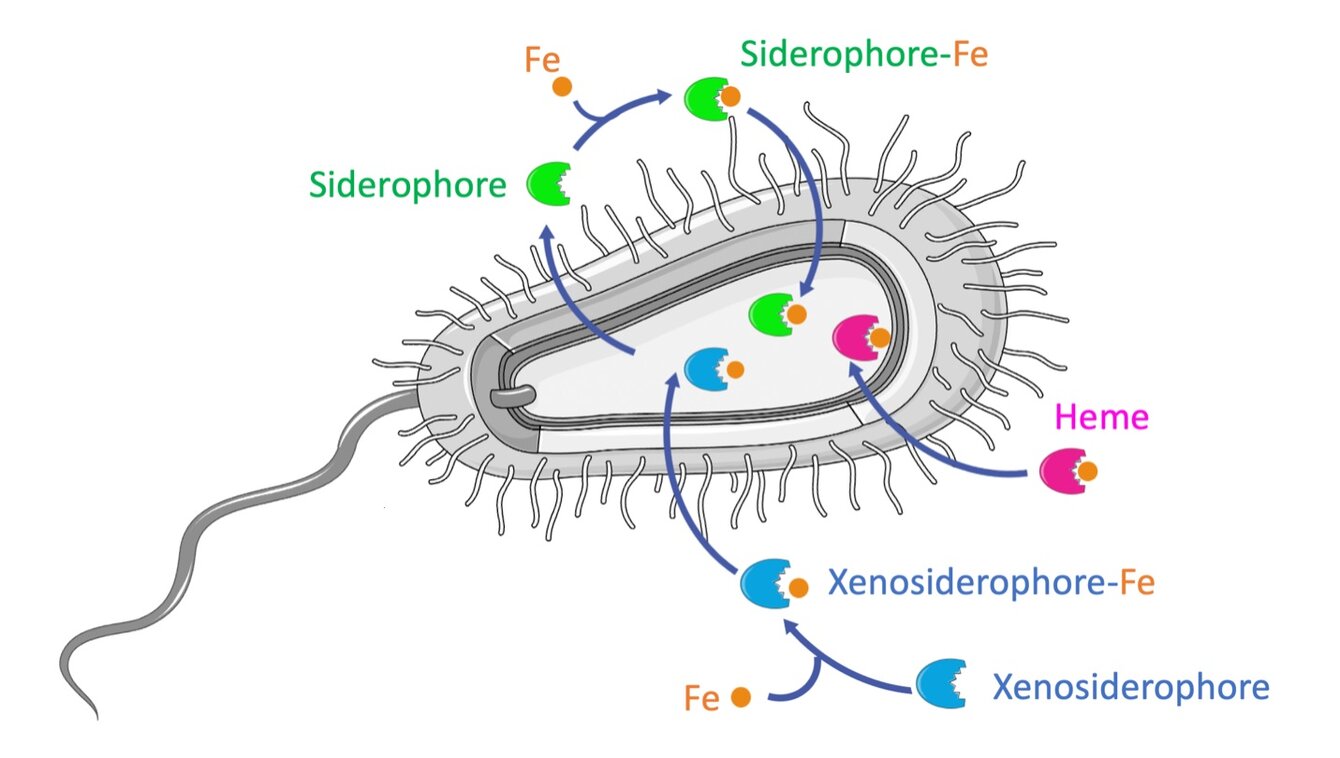
Notre équipe présente la particularité d’être composée de biochimistes, biologistes moléculaires, microbiologistes et chimistes organiciens. Ces compétences sont associées dans le cadre d’une approche pluridisciplinaire focalisée sur l’homéostasie du fer chez les bactéries.
Le fer est un nutriment essentiel aux organismes vivants car c’est un cofacteur de nombreuses enzymes impliquées dans des processus métaboliques clés comme la respiration cellulaire, la biosynthèse de nucléotides, la transcription et la réparation de l’ADN. Le fer étant faiblement biodisponible, les bactéries synthétisent des sidérophores pour capturer ce nutriment de leur environnement. Les sidérophores sont de petites molécules organiques ayant une très forte affinité pour le fer et qui, produites durant les infections, jouent un rôle clé dans la virulence. Notre modèle est Pseudomonas aeruginosa, un pathogène opportuniste qui peut utiliser au moins 20 voies différentes d’acquisition du fer.
Étude des mécanismes moléculaires impliqués dans l’assimilation du fer. Nous essayons de comprendre les interactions moléculaires entre les protéines impliquées dans l’acquisition du fer, leur organisation et distribution dans la paroi bactérienne et comment les machineries protéiques de transport permettent la translocation du métal. Nous nous intéressons également aux mécanismes moléculaires impliqués dans la régulation de l’expression des différentes voies d’acquisition du fer chez P. aeruginosa.
Définition de nouvelles cibles biologiques pour des antibiotiques innovants. P. aeruginosa est à l’origine de nombreuses infections nosocomiales et d’infections létales chez les malades atteints par la mucoviscidose. Ceux-ci présentent de plus en plus de résistance aux antibiotiques classiques. Dans ce contexte, les voies de transports du fer sont des cibles prometteuses pour concevoir une nouvelle génération d’antibiotiques. Partant des postulats que le fer est essentiel à la prolifération bactérienne au cours de l’infection et que les systèmes de transport du fer sont à la fois efficaces et sélectifs, deux stratégies sont développées :
- Synthèse d’inhibiteurs des protéines impliquées dans le transport du fer pour inhiber la prolifération bactérienne.
- Synthèse de conjugués sidérophore-antibiotique se comportant comme des « chevaux de Troie » capables d’utiliser les voies d’assimilation du fer pour s’introduire dans la bactérie. Les antibiotiques vectorisés dans cette approche sont à la fois des molécules approuvées mais incapables de pénétrer l’enveloppe de P. aeruginosa, ou des molécules bactéricides originales (complexes métalliques, peptides antibactériens).
Bioremédiation. Nous étudions également la spécificité des voies de transport du fer utilisant des sidérophores vis-à-vis de nombreux métaux toxiques. L’objectif est de valider l’utilisation des voies d’assimilation du fer dans de nouveaux procédés de bioremédiation d’effluents liquides ou de sols contaminés en métaux lourds.
Contact
Soutiens financiers

Membres de l'équipe
- Bureau : D309
- emmanuel.boutant[at]unistra.fr
- Bureau : Pharma/D307
- capucine.braillon[at]etu.unistra.fr
- Bureau : D312
- Bureau : D309
- olivier.cunrath[at]unistra.fr
- Bureau : D307
- p.fechter[at]unistra.fr
- Bureau : D316
- manon.ferry[at]etu.unistra.fr
- Bureau : D311
- anne.forster[at]unistra.fr
- Bureau : D311
- veronique.gasser[at]unistra.fr
- Bureau : D313
- valerie.geoffroy[at]unistra.fr
- Bureau : D341
- walid.hajeb[at]etu.unistra.fr
- Bureau : D341
- francoise.hoegy[at]unistra.fr
- Bureau : D307
- regine.janel[at]unistra.fr
Mezouarhi Chaimae
- Bureau : D341
- gaetan.mislin[at]unistra.fr
Nuvoli Nicolas
- Bureau : D316
- christos.paschalidis[at]etu.unistra.fr
- Bureau : D341
- silvaresende[at]unistra.fr
- Bureau : D312
- anne-eloise.revillot-schmidt[at]etu.unistra.fr
- Bureau : D341
- revoltissot[at]unistra.fr
- Bureau : D307
- rigouin[at]unistra.fr
- Bureau : D317
- schalk[at]unistra.fr
Silberreiss Pauline
- Bureau : D312
- f.volck[at]unistra.fr
- Bureau : D312
- virginie.will[at]etu.unistra.fr
- Bureau : D310
- i.yousfi[at]unistra.fr
Publications
Salmonella relies on siderophore exploitation at low pH. Microlife 7:uqaf041. doi: 10.1093/femsml/uqaf041.
Michaut M, Hoegy F, Steffen A, Contreras JM, Morice C, Plésiat P, Mislin GLA (2025). 1,2,3-Triazole‑gold(I)-triethylphosphine derivatives of nutrients as new antimicrobials against antibiotic resistant Gram-positive pathogens. Bioorg Med Chem Lett 125-126:130276. doi: 10.1016/j.bmcl.2025.130276.
Will V, Moynié L, Si Ahmed Charrier E, Le Bas A, Kuhn L, Volck F, Chicher J, Aksoy H, Madec M, Antheaume C, Mislin GLA, Schalk IJ (2025). Structure of the Outer Membrane Transporter FemA and Its Role in the Uptake of Ferric Dihydro-Aeruginoic Acid and Ferric Aeruginoic Acid in Pseudomonas aeruginosa. ACS Chem Biol 20(3):690-706. doi: 10.1021/acschembio.4c00820.
Schalk IJ (2025). Bacterial siderophores: diversity, uptake pathways and applications. Nat Rev Microbiol 23(1):24-40. doi: 10.1038/s41579-024-01090-6.
Renault J, Couchot J, Faucon A, Renaud JL, Mislin GLA, Plésiat P, Gaillard S (2024). 2-Thiophenyl–Isoquinoline Ir(III) Complex: A Promising Tool in Antipseudomonal Photodynamic Therapy under Red Irradiation. Eur J InorgChem e202300767. doi: 10.1002/ejic.202300767.
Faucon A, Renault J, Josts I, Couchot J, Renaud JL, Hoegy F, Plésiat P, Tidow H, Gaillard S, Mislin GLA (2024). Synthesis and antibacterial properties under blue LED light of conjugates between the siderophore desferrioxamine B (DFOB) and an Iridium(III) complex. Bioorg Med Chem 112:117842. doi: 10.1016/j.bmc.2024.117842.
Will V, Frey C, Normant V, Kuhn L, Chicher J, Volck F, Schalk IJ (2024). The role of FoxA, FiuA, and FpvB in iron acquisition via hydroxamate-type siderophores in Pseudomonas aeruginosa. Sci Rep 14(1):18795. doi: 10.1038/s41598-024-69152-6.
Manko H, Steffan T, Gasser V, Mély Y, Schalk I, Godet J (2024). PvdL Orchestrates the Assembly of the Nonribosomal Peptide Synthetases Involved in Pyoverdine Biosynthesis in Pseudomonas aeruginosa. Int J Mol Sci 25(11):6013. doi: 10.3390/ijms25116013.
Puja H, Bianchetti L, Revol-Tissot J, Simon N, Shatalova A, Nommé J, Fritsch S, Stote RH, Mislin GLA, Potier N, Dejaegere A, Rigouin C (2024). Biosynthesis of a clickable pyoverdine via in vivo enzyme engineering of an adenylation domain. Microb Cell Fact 23(1):207. doi: 10.1186/s12934-024-02472-4.
Will V, Gasser V, Kuhn L, Fritsch S, Heinrichs DE, Schalk IJ (2023). Siderophore specificities of the Pseudomonas aeruginosa TonB-dependent transporters ChtA and ActA. FEBS Lett 597(23):2963-2974. doi: 10.1002/1873-3468.14740.
Spragge F, Bakkeren E, Jahn MT, B N Araujo E, Pearson CF, Wang X, Pankhurst L, Cunrath O, Foster KR (2023). Microbiome diversity protects against pathogens by nutrient blocking. Science 382(6676):eadj3502. doi: 10.1126/science.adj3502.
Puja H, Mislin GLA, Rigouin C (2023). Engineering Siderophore Biosynthesis and Regulation Pathways to Increase Diversity and Availability. Biomolecules 13(6):959. doi: 10.3390/biom13060959.
Olshvang E, Fritsch S, Scholtyssek OC, Schalk IJ, Metzler-Nolte N (2023). Vectorization via Siderophores Increases Antibacterial Activity of K(RW)3 Peptides against Pseudomonas aeruginosa. Chemistry 29(50):e202300364. doi: 10.1002/chem.202300364.
Hubert T, Madec M, Schalk IJ (2023). Experimental and computational methods to highlight behavioural variations in TonB-dependent transporter expression in Pseudomonas aeruginosa versus siderophore concentration. Sci Rep 13(1):20015. doi: 10.1038/s41598-023-46585-z.
Forster A, Graulier G, Schalk IJ, Fechter P (2023). Improved engineering of Pseudomonas aeruginosa to study the adaptation of pyoverdine production under intra- or inter- specific bacterial competition. J Microbiol Methods 210:106753. doi: 10.1016/j.mimet.2023.106753.
Schalk IJ, Perraud Q (2023). Pseudomonas aeruginosa and its multiple strategies to access iron. Environ Microbiol 25:811-831. doi: 10.1111/1462-2920.16328.
Peukert C, Gasser V, Orth T, Fritsch S, Normant V, Cunrath O, Schalk IJ*, Brönstrup M* (2023). Trojan Horse Siderophore Conjugates Induce Pseudomonas aeruginosa Suicide and Qualify the TonB Protein as a Novel Antibiotic Target. J Med Chem 66:553-576. doi: 10.1021/acs.jmedchem.2c01489.
Fritsch S, Gasser V, Peukert C, Pinkert L, Kuhn L, Perraud Q, Normant V, Brönstrup M, Schalk IJ (2022). Uptake Mechanisms and Regulatory Responses to MECAM- and DOTAM-Based Artificial Siderophores and Their Antibiotic Conjugates in Pseudomonas aeruginosa. ACS Infect Dis 8(6):1134-1146. doi: 10.1021/acsinfecdis.2c00049.
Moynié L, Hoegy F, Milenkovic S, Munier M, Paulen A, Gasser V, Faucon AL, Zill N, Naismith JH, Ceccarelli M, Schalk IJ, Mislin GLA (2022). Hijacking of the enterobactin pathway by a synthetic catechol vector designed for oxazolidinone antibiotic delivery in Pseudomonas aeruginosa. ACS Infect Dis 8:1894-1904. doi: 10.1021/acsinfecdis.2c00202.
Lemare M, Puja H, David SR, Mathieu S, Ihiawakrim D, Geoffroy VA, Rigouin C (2022). Engineering siderophore production in Pseudomonas to improve asbestos weathering. Microb. Biotechnol 15:2351-2363. doi: 10.1111/1751-7915.14099.
Luscher A, Gasser V, Bumann D, Mislin GLA, Schalk IJ, Köhler T (2022). Plant-Derived Catechols Are Substrates of TonB-Dependent Transporters and Sensitize Pseudomonas aeruginosa to Siderophore-Drug Conjugates. mBio 13:e0149822. doi: 10.1128/mbio.01498-22.
Faucon AL, Hoegy F, Werle N, Gourlaouen C, Mislin GLA (2022). Dipyridylamine-acetamide (Dpaa): A primary amine protecting group orthogonally cleavable under acidic conditions in the presence of t-butyloxycarbonyl (Boc) and t-butylester. Tetrahedron Letters 96:153758. doi: 10.1016/j.tetlet.2022.153758.
Abdallah B, Seguin C, Aubert E, Ait BenHassou H, Sbabou L, Choulier L, Vonthron C, Schalk IJ, Mislin GLA, Fournel S, Pitchon V, Fechter P (2022). Past mastering of metal transformation enabled physicians to increase their therapeutic potential. J Trace Elem Med Biol 71:126926. doi: 10.1016/j.jtemb.2022.126926.
Roche B, Garcia-Rivera MA, Normant V, Kuhn L, Hammann P, Brönstrup M, Mislin GLA, Schalk IJ (2022). A role for PchHI as the ABC transporter in iron acquisition by the siderophore pyochelin in Pseudomonas aeruginosa. Environ Microbiol 24(2):866-877. doi: 10.1111/1462-2920.15811.
Perraud Q, Kuhn L, Fritsch S, Graulier G, Gasser V, Normant V, Hammann P, Schalk IJ (2021). Opportunistic use of catecholamine neurotransmitters as siderophores to access iron by Pseudomonas aeruginosa. Environ Microbiol 24(2):878-893. doi: 10.1111/1462-2920.15372.
Normant V, Kuhn L, Munier M, Hammann P, Mislin GLA, Schalk IJ (2021). How the Presence of Hemin Affects the Expression of the Different Iron Uptake Pathways in Pseudomonas aeruginosa Cells. ACS Infect Dis 8:183-196 (Cover page). doi: 10.1021/acsinfecdis.1c00525.
Kouijzer JJP, Lattwein KR, Beekers I, Langeveld SAG, Leon-Grooters M, Strub JM, Oliva E, Mislin GLA, de Jong N, van der Steen AFW, Klibanov AL, van Wamel WJB, Kooiman K (2021). Vancomycin-decorated microbubbles as a theranostic agent for Staphylococcus aureus biofilm. Int J Pharm 609:121154. doi: 10.1016/j.ijpharm.2021.121154.
Josts I, Veith K, Normant V, Schalk IJ, Tidow H (2021). Structural insights into a novel family of integral membrane siderophore reductases. Proc Natl Acad Sci USA 118(34):e2101952118. doi: 10.1073/pnas.2101952118.
Tetard A, Foley S, Mislin GLA, Brunel JM, Oliva E, Torrealba Anzola F, Zedet A, Cardey B, Pellequer Y, Ramseyer C, Plésiat P, Llanes C (2021). Negative Impact of Citral on Susceptibility of Pseudomonas aeruginosa to Antibiotics. Front Microbiol 12:709838.
Michaut M, Steffen A, Contreras JM, Morice C, Schalk IJ, Plésiat P, Mislin GLA (2021). 1,2,3-Triazole-gold(I)-triethylposphine derivatives active against resistant Gram-positive pathogens. Bioorg Med Chem Lett 40:127879. doi: 10.1016/j.bmcl.2021.127879.
Gasser V, Kuhn L, Hubert T, Aussel L, Hammann P, Schalk IJ (2021). The Esterase PfeE, the Achilles’ Heel in the Battle for Iron between Pseudomonas aeruginosa and Escherichia coli. Int J Mol Sci 22(6):2814. doi: 10.3390/ijms22062814.
David SR, Jaouen A, Ihiawakrim D, Geoffroy VA (2021). Biodeterioration of asbestos cement by siderophore-producing Pseudomonas. J Hazard Mater 403:123699. doi: 10.1016/j.jhazmat.2020.123699.
Roche B, Mislin GLA, Schalk IJ (2021). Identification of the fatty acid coenzyme-A ligase FadD1 as an interacting partner of FptX in the Pseudomonas aeruginosa pyochelin pathway. FEBS Letters 595(3):370-378. doi: 10.1002/1873-3468.14012.
David SR, Ihiawakrim D, Regis R, Geoffroy VA (2020). Efficiency of pyoverdines in iron removal from flocking asbestos waste: An innovative bacterial bioremediation strategy. J Hazard Mater 394:122532. doi: 10.1016/j.jhazmat.2020.122532.
David SR, Geoffroy VA (2020). A Review of Asbestos Bioweathering by Siderophore-Producing Pseudomonas: A Potential Strategy of Bioremediation. Microorganisms 8(12):1870. doi: 10.3390/microorganisms8121870.
Cunrath O, Graulier G, Carballido-Lopez A, Pérard J, Forster A, Geoffroy VA, Saint Auguste P, Bumann D, Mislin GLA, Michaud-Soret I, Schalk IJ, Fechter P (2020). The pathogen Pseudomonas aeruginosa optimizes the production of the siderophore pyochelin upon environmental challenges. Metallomics 12(12):2108-2120. doi: 10.1039/d0mt00029a.
Perraud Q, Cantero P, Munier M, Hoegy F, Zill N, Gasser V, Mislin GLA, Ehret-Sabatier L, Schalk IJ (2020). Phenotypic Adaptation of Pseudomonas aeruginosa in the Presence of Siderophore-Antibiotic Conjugates during Epithelial Cell Infection. Microorganisms 8(11):1820. doi: 10.3390/microorganisms8111820.
David SR, Ihiawakrim D, Regis R, Geoffroy VA (2020). Iron removal from raw asbestos by siderophores-producing Pseudomonas. J Hazard Mater 385:121563. doi: 10.1016/j.jhazmat.2019.121563.
Normant V, Josts I, Kuhn L, Perraud Q, Fritsch S, Hammann P, Mislin GLA, Tidow H, Schalk IJ (2020). Nocardamine-Dependent Iron Uptake in Pseudomonas aeruginosa: Exclusive Involvement of the FoxA Outer Membrane Transporter. ACS Chem Biol 15(10):2741-2751. doi: 10.1021/acschembio.0c00535.
Michaut M, Steffen A, Contreras JM, Morice C, Paulen A, Schalk IJ, Plésiat P, Mislin GLA (2020). Chryso-lactams:Gold(I) derivatives of ampicillin with specific activity against Gram-positive pathogens. Bioorg Med Chem Lett 30:127098. doi: 10.1016/j.bmcl.2020.127098.
Behrens HM, Lowe ED, Gault J, Housden NG, Kaminska R, Weber TM, Thompson CMA, Mislin GLA, Schalk IJ, Walker D, Robinson CV, Kleanthous C (2020). Pyocin S5 import into Pseudomonas aeruginosa reveals a generic mode of bacteriocin transport. mBio 11: e03230-19. doi: 10.1128/mBio.03230-19.
Manko H, Normant V, Perraud Q, Steffan T, Gasser V, Boutant E, Réal É, Schalk IJ, Mély Y, Godet J (2020) . FLIM-FRET Measurements of Protein-Protein Interactions in Live Bacteria. J Vis Exp. 162. doi.org/10.3791/61602.
David SR, Fritsch S, Forster A, Ihiawakrim D, Geoffroy VA (2020). Flocking asbestos waste, an iron and magnesium source for Pseudomonas. Sci Total Environ 709:135936. doi: 10.1016/j.scitotenv.2019.135936.
Perraud Q, Cantero P, Roche B, Gasser V, Normant VP, Kuhn L, Hammann P, Mislin GLA, Ehret-Sabatier L, Schalk IJ (2020). Phenotypic adaption of Pseudomonas aeruginosa by hacking siderophores produced by other microorganisms. Mol Cell Proteomics 19:589-607. doi: 10.1074/mcp.RA119.001829.
Schalk IJ, Rigouin C, Godet J (2020). An overview of siderophore biosynthesis among fluorescent Pseudomonads and new insights into their complex cellular organization. Environ Microbiol 22:1447-1466. doi: 10.1111/1462-2920.14937.
Bonneau A, Roche B, Schalk IJ (2020). Iron acquisition in Pseudomonas aeruginosa by the siderophore pyoverdine: an intricate interacting network including periplasmic and membrane proteins. Sci Rep. 10:120. doi: 10.1038/s41598-019-56913-x.
Gasser V, Malrieu M, Forster A, Mély Y, Schalk IJ, Godet J (2020). In cellulo FRET-FLIM and single molecule tracking reveal the supra-molecular organization of the pyoverdine bio-synthetic enzymes in Pseudomonas aeruginosa. Q Rev Biophys 53:e1. doi: 10.1017/S0033583519000155.
Vigouroux A, Aumont-Nicaise M, Boussac A, Marty L, Lo Bello L, Legrand P, Brillet K, Schalk IJ, Moréra S (2020). A unique ferrous iron binding mode is associated with large conformational changes for the transport protein FpvC of Pseudomonas aeruginosa. FEBS J 287:295-309. doi: 10.1111/febs.15004.
Fechter P, Cruz Da Silva E, Mercier MC, Noulet F, Etienne-Seloum N, Guenot D, Lehmann M, Vauchelles R, Martin S, Lelong-Rebel I, Ray AM, Seguin C, Dontenwill M, Choulier L (2019). RNA Aptamers Targeting Integrin α5β1 as Probes for Cyto- and Histofluorescence in Glioblastoma. Mol Ther Nucleic Acids 17:63-77. doi: 10.1016/j.omtn.2019.05.006.
Vu TH, Ha-Duong NT, Aubry A, Capton E, Fechter P, Plésiat P, Verbeke P, Serradji N (2019). In vitro activities of a new fluoroquinolone derivative highly active against Chlamydia trachomatis. Bioorg Chem 83:180-185. doi: 10.1016/j.bioorg.2018.10.033.
Loyaux-Lawniczak S, Vuilleumier S, Geoffroy VA (2019). Efficient Reduction of Iron Oxides by Paenibacillus spp. Strains Isolated from Tropical Soils. Geomicrobiology journal 36:423–432.
Carballido Lopez A, Cunrath O, Forster A, Pérard J, Graulier G, Legendre R, Varet H, Sismeiro O, Perraud Q, Pesset B, Saint Auguste P, Bumann D, Mislin GLA, Coppee JY, Michaud-Soret I, Fechter P, Schalk IJ (2019). Non-specific interference of cobalt with siderophore-dependent iron uptake pathways. Metallomics 11:1937-1951. doi: 10.1039/c9mt00195f.
Moynié L, Milenkovic S, Mislin GLA, Gasser V, Malloci G, Baco E, McCaughan RP, Page MGP, Schalk IJ, Ceccarelli M, Naismith JH (2019). The complex of ferric-enterobactin with its transporter from Pseudomonas aeruginosa suggests a two-site model. Nat Commun 10:3673. doi: 10.1038/s41467-019-11508-y.
Briard B, Mislin GLA, Latgé JP, Beauvais A (2019). Interactions between Aspergillus fumigatus and Pulmonary Bacteria: Current State of the Field, New Data, and Future Perspective. J Fungi 5:48. doi: 10.3390/jof5020048.
Fechter P (2018). Mapping Changes in Cell Surface Protein Expression Through Selective Labeling of Live Cells. Methods Mol Biol 1737:119-127. doi: 10.1007/978-1-4939-7634-8_8.
Perraud Q, Moynié L, Gasser V, Munier M, Godet J, Hoegy F, Mély Y, Mislin GLA, Naismith JH, Schalk IJ (2018). A Key Role for the Periplasmic PfeE Esterase in Iron Acquisition via the Siderophore Enterobactin in Pseudomonas aeruginosa.ACS Chem Biol 13:2603-2614. doi: 10.1021/acschembio.8b00543.
Schalk IJ (2018). Siderophore-antibiotic conjugates: exploiting iron uptake to deliver drugs into bacteria. Clin Microbiol Infect24:801-802. doi: 10.1016/j.cmi.2018.03.037.
Schalk IJ (2018). A Trojan-Horse Strategy Including a Bacterial Suicide Action for the Efficient Use of a Specific Gram-Positive Antibiotic on Gram-Negative Bacteria. J Med Chem 61:3842-3844. doi: 10.1021/acs.jmedchem.8b00522.
Sauvageot E, Elie M, Gaillard S, Daniellou R, Fechter P, Schalk IJ, Gasser V, Renaud JL, Mislin GLA (2017). Antipseudomonal activity enhancement of luminescent iridium(iii) dipyridylamine complexes under visible blue light. Metallomics 9:1820-1827. doi: 10.1039/c7mt00262a.
Paulen A, Hoegy F, Roche B, Schalk IJ, Mislin GLA (2017). Synthesis of conjugates between oxazolidinone antibiotics and a pyochelin analogue. Bioorg Med Chem Lett 27:4867-4870. doi: 10.1016/j.bmcl.2017.09.039.
Schalk IJ, Mislin GLA (2017). Bacterial Iron Uptake Pathways: Gates for the Import of Bactericide Compounds. J Med Chem 60:4573-4576. doi: 10.1021/acs.jmedchem.7b00554.
Ganne G, Brillet K, Basta B, Roche B, Hoegy F, Gasser V, Schalk IJ (2017). Iron Release from the Siderophore Pyoverdine in Pseudomonas aeruginosa Involves Three New Actors: FpvC, FpvG, and FpvH. ACS Chem Biol 12:1056-1065. doi: 10.1021/acschembio.6b01077.
Molina L, Geoffroy VA, Segura A, Udaondo Z, Ramos JL (2016). Iron uptake analysis in a set of clinical isolates of Pseudomonas putida. Front Microbiol 7:2100. doi: 10.3389/fmicb.2016.02100.
Cunrath O, Geoffroy VA, Schalk IJ (2016). Metallome of Pseudomonas aeruginosa : a role for siderophores. Environ Microbiol 18:3258-3267. doi: 10.1111/1462-2920.12971.
Gasser V, Baco E, Cunrath O, August PS, Perraud Q, Zill N, Schleberger C, Schmidt A, Paulen A, Bumann D, Mislin GL, Schalk IJ (2016). Catechol siderophores repress the pyochelin pathway and activate the enterobactin pathway in Pseudomonas aeruginosa : an opportunity for siderophore-antibiotic conjugates development. Environ Microbiol 18:819-32. doi: 10.1111/1462-2920.13199.
Schalk IJ, Cunrath O (2016). An overview of the biological metal uptake pathways in Pseudomonas aeruginosa. Environ Microbiol 18:3227-3246. doi: 10.1111/1462-2920.13525.
Catekl-Ferreira M, Marti S, Guillon L, Jara L, Coadou G, Molle V, Bouffartigues E, Bou G, Schalk I, Jouenne T, Vila-Farrés X, Dé E (2016). The outer membrane porin OmpW of Acinetobacter baumannii is involved in iron uptake and colistin binding. FEBS Lett 590: 224-31.
Briard B, Bomme P, Lechner BE, Mislin GLA, Lair V, Prévost MC, Latgé JP, Haas H, Beauvais A (2015). Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci Rep 5:8220.
Cunrath O, Gasser V, Hoegy F, Reimmann C, Guillon L, Schalk IJ (2015). A cell biological view of the siderophore pyochelin iron uptake pathway in Pseudomonas aeruginosa. Environ Microbiol 17:171-185.
Ferret C, Cornu JY, Elhabiri M, Sterckeman T, Braud A, Jezequel K, Lollier M, Lebeau T, Schalk IJ, Geoffroy VA (2015). Effect of pyoverdine supply on cadmium and nickel complexation and phytoavailability in hydroponics. Environ Sci Pollut Res 22:2106-2116.
Gasser V, Guillon L, Cunrath O, Schalk IJ (2015). Cellular organization of siderophore biosynthesis in Pseudomonas aeruginosa : Evidence for siderosomes. J Inorg Biochem 148:27-34.
Paulen A, Gasser V, Hoegy F, Perraud Q, Pesset B, Schalk IJ, Mislin GL (2015). Synthesis and antibiotic activity of oxazolidinone–catechol conjugates against Pseudomonas aeruginosa. Org Biomol Chem 13:11567-11579.
Vaulont S, Schalk I (2015). Roles of bacterial and mammalian siderophores in host-pathogen interactions. Med Sci 31:756-63.
Baco E, Hoegy F, Schalk IJ, Mislin GL (2014). Diphenyl-benzo[1,3]dioxole-4-carboxylic acid pentafluorophenyl ester : a convenient catechol precursor in the synthesis of siderophore vectors suitable for antibiotic Trojan horse strategies. Org Biomol Chem 12:749-757.
Cornu JY, Elhabiri M, Ferret C, Geoffroy VA, Jezequel K, Leva Y, Lollier M, Schalk IJ, Lebeau T (2014). Contrasting effects of pyoverdine on the phytoextraction of Cu and Cd in a calcareous soil. Chemosphere 103:212-219.
Ferret C, Sterckeman T, Cornu J-Y, Gangloff S, Schalk IJ, Geoffroy VA (2014). Siderophore promoted dissolution of smectite by fluorescent Pseudomonas. Environ Microbiol Reports 6:459-467.
Kizhakkepowathial KU, Prakasan P, Geoffroy VA, Doble M, Sailas B (2014). Pseudomonas aeruginosa BUP2- a novel strain isolated form Malabari goat produces type 2 pyoverdine. Advances in Bioscience and biotechnology 5:874-885.
Hoegy F, Mislin GLA, Schalk IJ (2014). Pyoverdine and pyochelin measurements. Methods Mol Biol 1149:293-301.
Hoegy F, Schalk IJ (2014). Monitoring iron uptake by siderophores. Methods Mol Biol 1149:337-346.
Maspoli A, Wenner N, Mislin GL, Reimmann C (2014). Functional analysis of pyochelin-/enantiopyochelin-related genes from a pathogenicity island of Pseudomonas aeruginosa strain PA14. Biometals 27:559-573.
Mislin GL, Schalk IJ (2014). Siderophore-dependent iron uptake systems as gates for antibiotic Trojan horse strategies against Pseudomonas aeruginosa. Metallomics 6:408-420.
Noël S, Hoegy F, Rivault F, Rognan D, Schalk IJ, Mislin GL (2014). Synthesis and biological properties of thiazole-analogues of pyochelin, a siderophore of Pseudomonas aeruginosa. Bioorg Med Chem Lett 24:132-135.
Unni KN, Priji P, Geoffroy VA, Doble M, Benjamin S (2014). Pseudomonas aeruginosa BUP2 - a novel strain isolated from Malabari goat produces type 2 pyoverdine. Adv Biosci Biotechnol 5:874-885.
Cornu JY, Elhabiri M, Ferret C, Geoffroy VA, Jezequel K, Leva Y, Lollier M, Schalk IJ, Lebeau T (2013). Contrasting effects of pyoverdine on the phytoextraction of Cu and Cd in a calcareous soil. Chemosphere 103:212-9.
Guillon L, Altenburger S, Graumann PL, Schalk IJ (2013). Deciphering Protein Dynamics of the Siderophore Pyoverdine Pathway in Pseudomonas aeruginosa. PLoS One 8:e79111.
Julou T, Mora T, Guillon L, Croquette V, Schalk IJ, Bensimon D, Desprat N (2013). Cell-cell contacts confine public goods diffusion inside Pseudomonas aeruginosa clonal microcolonies. Proc Natl Acad Sci U S A 31:12577-12582.
Schalk IJ, Guillon L (2013). Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa : implications for metal homeostasis. Environ Microbiol 15:1661-1673.
Schalk IJ (2013). Innovation and originality in the strategies developed by bacteria to get access to iron. Chembiochem 14:293-294.
Schalk IJ, Guillon L (2013). Fate of ferrisiderophores after import across bacterial outer membranes : different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids 5:1267-1277.
Brandel J, Humbert N, Elhabiri M, Schalk IJ, Mislin GL, Albrecht-Garry AM (2012). Pyochelin, a siderophore of Pseudomonas aeruginosa : Physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans 41:2820-2834.
Brillet K, Ruffenach F, Adams H, Journet L, Gasser V, Hoegy F, Guillon L, Hannauer M, Page A, Schalk IJ (2012). An ABC Transporter with Two Periplasmic Binding Proteins Involved in Iron Acquisition in Pseudomonas aeruginosa. ACS Chem Biol 7:2036-2045.
Guillon L, El Mecherki M, Altenburger S, Graumann PL, Schalk IJ (2012). High cellular organization of pyoverdine biosynthesis in Pseudomonas aeruginosa : clustering of PvdA at the old cell pole. Environ Microbiol 14:1982-1994.
Hannauer M, Braud A, Hoegy F, Ronot P, Boos A, Schalk IJ (2012). The PvdRT-OpmQ efflux pump controls the metal selectivity of the iron uptake pathway mediated by the siderophore pyoverdine in Pseudomonas aeruginosa. Environ Microbiol 14:1696-1708.
Hannauer M, Schafer M, Hoegy F, Gizzi P, Wehrung P, Mislin GL, Budzikiewicz H, Schalk IJ (2012). Biosynthesis of the pyoverdine siderophore of Pseudomonas aeruginosa involves precursors with a myristic or a myristoleic acid chain. FEBS Lett 586:96-101.
Noël S, Rivault F, Schalk IJ, Mislin GLA (2012). Chimie des sidérophores : vers de nouvelles molécules pour une santé de fer ? Partie I. Stratégies innovantes pour la conception d'antibiotiques. Arch Sci Nat Phys Math NS 46:123-151.
Schalk IJ., Mislin GL, Brillet K (2012). Structure, Function and Binding Selectivity and Stereoselectivity of Siderophore–Iron Outer Membrane Transporters. Curr Top Membr 69:37-66.
Brillet K, Reimmann C, Mislin GL, Noël S, Rognan D, Schalk IJ, Cobessi D (2011). Pyochelin enantiomers and their outer-membrane siderophore transporters in fluorescent pseudomonads : structural bases for unique enantiospecific recognition. J Am Chem Soc 133:16503-9.
Nader M, Journet L, Meksem A, Guillon L, Schalk IJ (2011). Mechanism of ferripyoverdine uptake by Pseudomonas aeruginosa outer membrane transporter FpvA : no diffusion channel formed at any time during ferrisiderophore uptake. Biochemistry 50:2530–2540.
Noel S, Gasser V, Pesset B, Hoegy F, Rognan D, Schalk IJ, Mislin GL (2011). Synthesis and biological properties of conjugates between fluoroquinolones and a N3’’-functionalized pyochelin. Org Biomol Chem 9:8288-8300.
Noel S, Guillon L, Schalk IJ, Mislin GL (2011). Synthesis of fluorescent probes based on the pyochelin siderophore scaffold. Org Lett 13:844–847.
Rodriguez-Lucena D, Schalk IJ, Mislin GL (2011). Synthesis of gemini triethylene-tetramine bridged bis-tridentate iron(III) chelators. Tetrahedron 67:2149-2154.
Schalk IJ, Hannauer M, Braud A (2011). New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol 13:2844-54.
Yang B, Hoegy F, Mesini PJ, Mislin GL, Schalk IJ (2011). Terbium, a fluorescent probe for investigation of siderophore pyochelin interactions with its outer membrane transporter FptA. J Inorg Biochem 105:1293-1298.
Braud A, Geoffroy V, Hoegy F, Mislin GL, Schalk IJ (2010). Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Environ Microbiol Rep 2:419–425.
Hannauer M, Barda Y, Mislin GL, Shanzer A, Schalk IJ (2010). The ferrichrome uptake pathway in Pseudomonas aeruginosa involves an iron release mechanism with acylation of the siderophore and a recycling of the modified desferrichrome. J Bacteriol 192:1212-20.
Hannauer M, Yeterian E, Martin LW, Lamont IL, Schalk IJ (2010). An efflux pump is involved in secretion of newly synthesized siderophore by Pseudomonas aeruginosa. FEBS Lett 584:4751-5.
Hoegy F, Gwynn MN, Schalk IJ (2010). Susceptibility of Pseudomonas aeruginosa to catechol-substituted cephalosporin is unrelated to the pyochelin-Fe transporter FptA.? Amino Acids 38:1627-9.
Rodriguez-Lucena D, Gaboriau F, Rivault F, Schalk IJ, Lescoat G, Mislin GL (2010). Synthesis and biological properties of iron chelators based on a bis-2-(2-hydroxy-phenyl)-thiazole-4-carboxamide or -thiocarboxamide (BHPTC) scaffold. Bioorg Med Chem 18:689-95.
Yétérian E, Martin LW, Guillon L, Journet L, Lamont IL, Schalk IJ (2010). Synthesis of the siderophore pyoverdine in Pseudomonas aeruginosa involves a periplasmic maturation. Amino Acids 38:1447-59.
Yétérian E, Martin LW, Lamont IL, Schalk IJ (2010). An efflux pump is required for siderophore recycling by Pseudomonas aeruginosa. Environ Microbiol Rep 2:412-8.
Braud A, Hannauer M, Mislin GL, Schalk IJ (2009). The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J Bacteriol 191:3517-25.
Braud A, Hoegy F, Jézéquel K, Lebeau K, Schalk IJ (2009). New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ Microbiol 11:1079-91.
Greenwald J, Nader M, Célia H, Gruffaz C, Geoffroy V, Meyer JM, Schalk IJ, Pattus F (2009). FpvA bound to non-cognate pyoverdines : molecular basis of siderophore recognition by an iron transporter. Mol Microbiol 72:1246-59.
Hoegy F, Lee X, Noel S, Rognan D, Mislin GL, Reimmann C, Schalk IJ (2009). Stereospecificity of the siderophore pyochelin outer membrane transporters in fluorescent Pseudomonads. J Biol Chem 284:14949-57.
Schalk IJ, Lamont LA, Cobessi D (2009). Structure-function relationships in the bifunctional ferri-siderophore FpvA receptor from Pseudomonas aeruginosa. Biometals 22:671-8.
Aouad G, Crovisier JL, Damidot D, Stille P, Hutchens E, Mutterer J, Meyer JM, Geoffroy V (2008). Interaction between municipal solid waste incinerator bottom ash and Pseudomonas aeruginosa. Sci Total Environ 393:385-393.
Schalk IJ (2008). Metal trafficking via siderophores in Gram-negative bacteria : specificities and characteristics of the pyoverdine pathway. J Inorg Biochem 102:1159-69.
Greenwald J, Hoegy F, Nader M, Journet J, Mislin GL, Graumann PL, Schalk IJ (2007). Real time fluorescent resonance energy transfer visualization of ferric pyoverdine uptake in Pseudomonas aeruginosa. A role for ferrous iron. J Biol Chem 282:2987-95.
Nader M, Dobbelaere W, Vincent M, Journet L, Adams H, Cobessi D, Gallay J, Schalk IJ (2007). Identification of residues of FpvA involved in the different steps of the Pvd-Fe uptake in Pseudomonas aeruginosa. Biochemistry 46:11707-17.
Rivault F, Liébert C, Burger A, Hoegy F, Abdallah MA, Schalk IJ, Mislin GL (2007). Synthesis of pyochelin-norfloxacin conjugates. Bioorg Med Chem Lett 17:640-4.
Youard ZA, Mislin GL, Majcherczyk PA, Schalk IJ, Reimmann C (2007). Pseudomonas fluorescens CHA0 produces enantio-pyochelin, the optical antipode of the Pseudomonas aeruginosa siderophore pyochelin. J Biol Chem 282:35546-53.

