Summary
Research interests
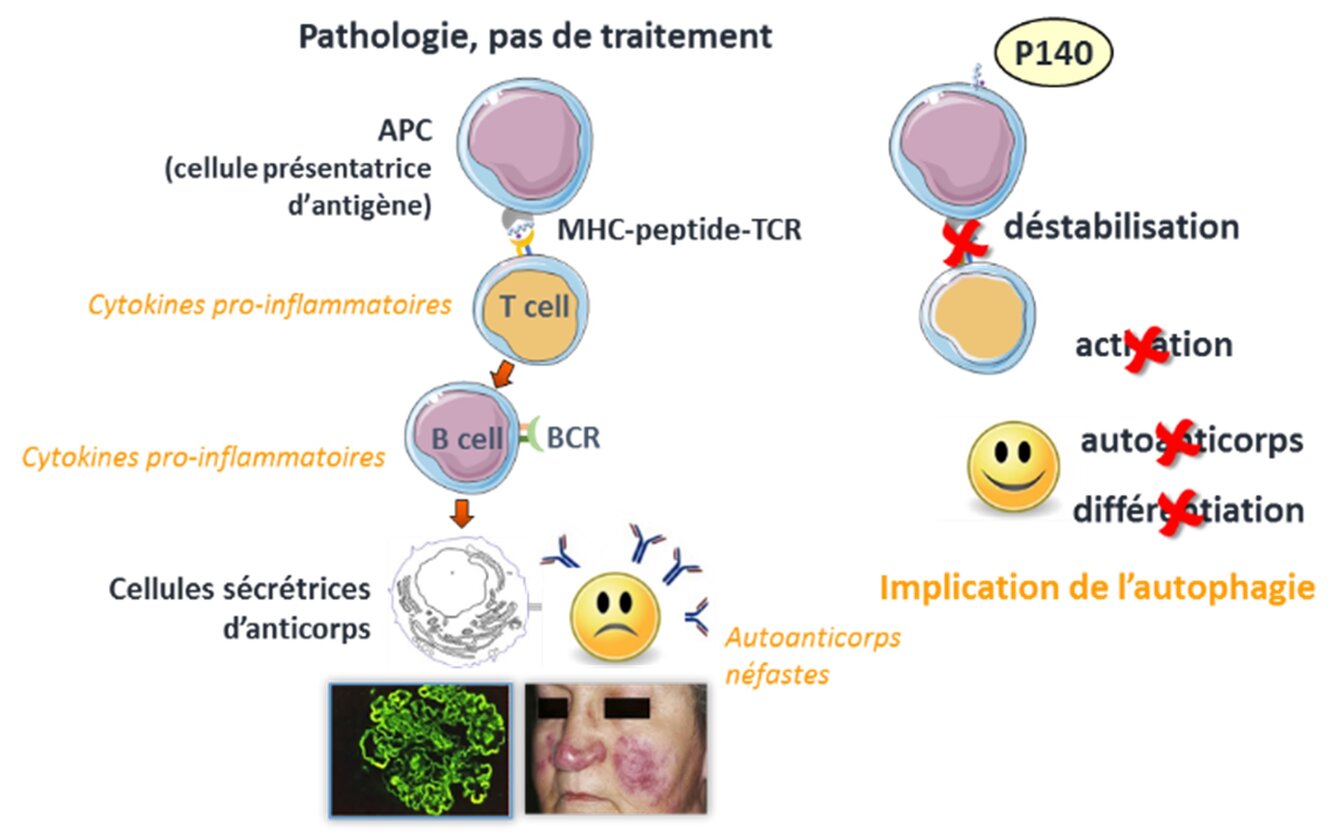
Our team focuses its research activities on the abnormal immune responses directed against the body's own tissues in autoimmune diseases, mainly on systemic lupus erythematosus (SLE), and on the discovery of new drug molecules designed to specifically immunoregulate these diseases.
SLE is a chronic multigenic disease whose etiology remains largely unknown. While the use of corticosteroids and immunosuppressants has allowed to provide much better patient care, these drugs have deleterious side effects, contraindications and some toxicity that can contribute to the morbidity of the disease. A few years ago, our group discovered a phosphopeptide, called P140, which shows protective effects in MRL/lpr mice that develop a disease similar to SLE. In a multicenter, randomized, placebo-controlled Phase IIb study, P140/LupuzorTM was shown to be free of side effects and met its primary efficacy endpoints, confirming preclinical data in lupus mice. It is currently in Phase III clinical trials.
The P140 peptide binds to and decreases the expression of the chaperone protein HSPA8/HSC70, which we found to be increased in lupus. This interaction, demonstrated both in vitro and in vivo, destabilizes the binding of HSPA8 to other chaperone proteins and affects its biological activity. When administered to MRL/lpr mice, P140 reduces hyperactivated autophagic flux in antigen-presenting B cells, with an accumulation of macroautophagy markers and a blockade of excessive chaperone-mediated autophagy (CMA) processing. In mice and lupus patients, P140 corrects a number of lysosome defects that we have discovered and decreases the abnormally high expression of major histocompatibility complex (MHC) molecules. All these effects contribute to reducing the hyperactivation of T and B cell autoreactivity and, ultimately, the production of pathogenic autoantibodies that are deposited in tissues creating a painful inflammatory state. P140 has demonstrated beneficial effects in animal models mimicking other inflammatory diseases as diverse as Sjögren's disease, inflammatory bowel disease (IBD), periodontitis, gout and asthma.
Our ongoing studies are particularly focused on:
At the cellular and molecular level, on lysosomal autophagy processes that appear abnormal in inflammatory conditions and prove to be decisive hotspots in the pathology of acute and chronic inflammatory conditions.
At the pathophysiological level, in lupus and other models of inflammation, on the clinical alterations that gradually emerge, including microbiome alterations, in relation to autophagy defects.
More broadly, on peptide strategies developed with the aim of specifically modulating autoreactivity, restoring immune tolerance and reversing the course of diseases in mouse models of chronic inflammatory pathologies, including neurological/neurodegenerative and metabolic diseases (MASH, obesity). Emphasis is placed on the possible abscopal effect in the mode of action of P140.
In parallel, we are developing innovative strategies to administer therapeutic peptides non-invasively using various nanostructures for their delivery. Efforts will also be devoted to theranostic issues with the development of specific chips to identify responders from non-responders to peptide treatment, and peptide tracking using new imaging methods on living individuals.
Contact
The team is based at the Institut de Science et d’Ingénierie Supramoléculaires (ISIS), 8 Allée Gaspard Monge, 67000 Strasbourg.
Pr Sylviane Muller
Pr Philippe Georgel
Funding

Team members
El Kaakour Lara
- Bureau : ISIS / Reims URCA 7369 MEDyC
- lelkaakour[at]unistra.fr
- Bureau : ISIS / 230
- pgeorgel[at]unistra.fr
- Bureau : ISIS / 231
- joffrey.mary[at]etu.unistra.fr
- Bureau : ISIS / 231
- dylan.mastrippolito[at]etu.unistra.fr
- Bureau : ISIS / 206
- sylviane.muller[at]unistra.fr
- Bureau : ISIS / 230
- talamini[at]unistra.fr
- Bureau : ISIS / 231
- cverdot[at]unistra.fr
Publications
Talamini L, Fonseca DLM, Kanduc D, Chaloin O, Verdot C, Galmiche C, Dotan A, Filgueiras IS, Borghi MO, Meroni PL, Gavrilova NY, Ryabkova VA, Churilov LP, Halpert G, Lensch C, Thurner L, Fong SW, Ng LFP, Rénia L, Young BE, Lye DC, Lozano JM, Cabral-Marques O, Shoenfeld Y, Muller S (2025). Long COVID-19 autoantibodies and their potential effect on fertility. Front Immunol 16:1540341. doi: 10.3389/fimmu.2025.1540341.
Kamalanathan AS, Agarwal V, Talamini L, Muller S (2025). Autophagy in myositis, a dysregulated pathway, and a target for therapy. Autoimmun Rev 24(7):103817. doi: 10.1016/j.autrev.2025.103817.
Brady S, Poulton J, Muller S (2024). Inclusion body myositis: Correcting mitochondfrial and lysosomal autophagy impairment as a potential therapeutic strategy. Autoimmun Rev 23(11):103644. doi: 10.1016/j.autrev.2024.103644.
Maujean T, Ramanoudjame SM, Riché S, Le Guen C, Boisson F, Muller S, Bonnet D, Gulea M, Marchand P (2024). Hetero-Diels-Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods. Molecules 29(13):3198. doi: 10.3390/molecules29133198.
Karonitsch T, Saferding V, Kieler M, von Dalwigk K, Tosevska A, Heller G, Dellinger M, Niederreiter B, Kartnig F, Steiner CW, Georgel P, Kiener HP, Smolen JS, Korb-Pap A, Bonelli M, Aletaha D, Blüml S (2024). Amino Acids Fueling Fibroblast-Like Synoviocyte Activation and Arthritis By Regulating Chemokine Expression and Leukocyte Migration. Arthritis Rheumatol 76(4):531-540. doi: 10.1002/art.42759.
Rafiq S, Mungure I, Banz Y, Niklaus NJ, Kaufmann T, Müller S, Jacquel A, Robert G, Auberger P, Torbett BE, Muller S, Tschan MP, Humbert M (2024). HSPA8 Chaperone Complex Drives Chaperone-Mediated Autophagy Regulation in Acute Promyelocytic Leukemia Cell Differentiation. Pharmacology 109(4):216-230. doi: 10.1159/000537864.
Bonam SR, Mastrippolito D, Georgel P, Muller S (2024). Pharmacological targets at the lysosomal autophagy-NLRP3 inflammasome crossroads. Trends Pharmacol Sci 45(1):81-101. doi: 10.1016/j.tips.2023.11.005.
Lasalo M, Jauffrais T, Georgel P, Matsui M (2024). Marine Microorganism Molecules as Potential Anti-Inflammatory Therapeutics. Mar Drugs 22(9):405. doi: 10.3390/md22090405.
Adiguzel Y, Mahroum N, Muller S, Blank M, Halpert G, Shoenfeld Y (2023). Shared Pathogenicity Features and Sequences between EBV, SARS-CoV-2, and HLA Class I Molecule-binding Motifs with a Potential Role in Autoimmunity. Clin Rev Allergy Immunol 65:206-230. doi: 10.1007/s12016-023-08962-4.
Renaudineau Y, Muller S, Hedrich CM, Chauveau D, Belliere J, De Almeida S, Damoiseaux J, Scherlinger M, Guery JC, Sailler L, Bost C (2023). Immunological and translational key challenges in systemic lupus erythematosus: a symposium update. J Transl Autoimmun 6:100199. doi: 10.1016/j.jtauto.2023.100199.
Muller S (2023). The abscopal effect: Implications for drug discovery in autoimmunity. Autoimmun Rev 22:103315. doi: 10.1016/j.autrev.2023.103315.
Jacotot E, Talamini L, Bonam SR, Vieira AT, Fremeaux-Bacchi V, Radic M, Dragon-Durey MA, Lozano JM, Saia RS, Muller S (2023). Innate immune responses in COVID-19. In Autoimmunity, COVID-19, Post-COVID19 syndrome and COVID-19 vaccination, 1st Edition (Eds: Shoenfeld Y, Dotan A); chapter 3 pages 63-128. doi: 10.1016/B978-0-443-18566-3.00041-4.
Dotan A, Kanduc D, Muller S, Shoenfeld Y (2023). SARS-CoV-2, fertility-related autoantibodies and reproductive injury. In Autoimmunity, COVID-19, Post-COVID19 syndrome and COVID-19 vaccination, 1st Edition (Eds: Shoenfeld Y, Dotan A); chapter 29 pages 595-601. doi: 10.1016/B978-0-443-18566-3.00024-4.
Gros F, Muller S (2023). The role of lysosomes in metabolic and autoimmune diseases. Nat Rev Nephrol 19(6):366-383. doi: 10.1038/s41581-023-00692-2.
Lozano JM, Muller S (2023). Monkeypox: Potential vaccine development strategies. Trends Pharmacol Sci 44:15-19. doi: 10.1016/j.tips.2022.10.005.
Georgel P, Lebouvier N, Matsui M (2023). Editorial: Exploring the unique biodiversity of the Western Pacific to identify novel anti-infectious and anti-inflammatory compounds of natural origin. Front Pharmacol 14:1154627. doi: 10.3389/fphar.2023.1154627.
Lathuillère C, Georgel P (2023). Recent progresses in innate neuro-immunology hold great promises for the management of inflammatory diseases. Brain Behavior and Immunity Integrative Volume 2, 100005. doi: 10.1016/j.bbii.2023.100005.
De Cauwer A, Pichot A, Molitor A, Stemmelen T, Carapito R, Bahram S, Georgel P (2023). Measuring the transcriptome-wide effects of aging on murine adipocytes using RNAseq. STAR Protoc 4(3):102397. doi: 10.1016/j.xpro.2023.102397.
Brun S, De Sèze J & Muller S (2022). CIDP: Current treatments and identification of targets for future specific therapeutic intervention. Immuno 2:118-131. doi: 10.3390/immuno2010009.
Galvão I, Mastrippolito D, Talamini L, Aganetti M, Rocha V, Verdot C, Mendes V, de Oliveira VLS, Braga AD, Martins VD, de Faria AMC, Amaral FA, Georgel P, Vieira AT, Muller S (2022). The therapeutic effect of phosphopeptide P140 attenuates inflammation induced by uric acid crystals in gout arthritis mouse model. Cells 11:3709. doi: 10.3390/cells11233709.
Thurner L, Fadle N, Regitz E, Preuss KD, Neumann F, Cetin O, Schormann C, Hoffmann MC, Herr C, Kheiroddin P, Rixecker TM, Bals R, Muller S, Thurner B, Kessel C, Kabesch M, Bewarder M, Heyne K, Lensch C, Kos IA (2022). Autoantibodies against SUMO1-DHX35 in long-COVID. J Transl Autoimmun 5:100171. doi: 10.1016/j.jtauto.2022.100171.
Akiyama K, Aung KT, Talamini L, Huck O, Kuboki T, Muller S (2022). Therapeutic effects of peptide P140 in a mouse periodontitis model. Cell Mol Life Sci 79:518. doi: 10.1007/s00018-022-04537-2.
Radic M, Muller S (2022). LL-37, a Multi-Faceted Amphipathic Peptide Involved in NETosis. Cells 11(15):2463. doi: 10.3390/cells11152463.
Schall N, Talamini L, Wilhelm M, Jouvin-Marche E, Muller S (2022). P140 peptide leads to clearance of autoreactive lymphocytes and normalizes immune response in lupus-prone mice. Front Immunol 13:904669. doi: 10.3389/fimmu.2022.904669.
Retnakumar SV, Geesala R, Bretin A, Tourneur-Marsille J, Ogier-Denis E, Maretzky T, Nguyen HTT, Muller S (2022) Targeting the endo-lysosomal autophagy pathway to treat inflammatory bowel diseases. J Autoimmun128:102814. doi: 10.1016/j.jaut.2022.102814.
Gay N, van Gemert C, Merilles OE Jr, Georgel P (2022). Pacific island nations face an urgent need for actions and future research on COVID-19. Lancet Reg Health West Pac 18:100326. doi: 10.1016/j.lanwpc.2021.100326.
De Cauwer A, Loustau T, Erne W, Pichot A, Molitor A, Stemmelen T, Carapito R, Orend G, Bahram S, Georgel P (2022). Dicer1 deficient mice exhibit premature aging and metabolic perturbations in adipocytes. iScience 25(10):105149. doi: 10.1016/j.isci.2022.105149.
Bonam SR, Tranchant C, Muller S (2021). Autophagy-Lysosomal Pathway as Potential Therapeutic Target in Parkinson’s Disease. Cells 10:3547. doi: 10.3390/cells10123547.
Schall N, Daubeuf F, Marsol C Frossard N, Bonnet D, Galzi JL, Muller S (2021). A selective neutraligand for CXCL12/SDF-1a with beneficial regulatory functions in MRL/lpr lupus prone mice. Front Pharmacol 12:752194. doi: 10.3389/fphar.2021.752194.
Daubeuf F, Schall N, Petit-Demoulière N, Frossard N, Muller S (2021). An autophagy modulator peptide prevents lung function decrease and corrects established inflammation in murine models of airway allergy. Cells 10:2468. doi: 10.3390/cells10092468.
Rafiq S, McKenna SL, Muller S, Tschan MP, Humbert M (2021). Lysosomes in acute myeloid leukemia: potential therapeutic targets? Leukemia. doi: 10.1038/s41375-021-01388-x.
Dotan A, Kanduc D, Muller S, Makatsariya A, Shoenfeld Y (2021). Molecular mimicry between SARS-CoV-2 and the female reproductive system. Am J Reprod Immunol 10.1111/aji.13494. doi: 10.1111/aji.13494.
Wilhelm M, Bonam SR, Schall N, Bendorius M, Korganow AA, Lumbroso C, Muller S (2021). Implication of a lysosomal antigen in the pathogenesis of lupus erythematosus. J Autoimmun 120:102633. doi: 10.1016/j.jaut.2021.102633.
Talamini L, Matsuura E, De Cola L, Muller S (2021). Immunologically inert nanostructures as selective therapeutic tools in inflammatory diseases. Cells 10(3):707. doi: 10.3390/cells10030707.
Klionsky DJ et al. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17:1-382. doi: 10.1080/15548627.2020.1797280.
Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y (2021). The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20(4):102792. doi: 10.1016/j.autrev.2021.102792.
Mariotte A, Bernardi L, Macquin C, DeCauwer A, Kotova I, Blüml S, Noël D, Scanu A, Punzi L, Carapito C, Sibilia J, Bahram S, Georgel P (2021). NKG2D ligands in inflammatory joint diseases: analysis in human samples and mouse models. Clin Exp Rheumatol 39(5):982-987. doi: 10.55563/clinexprheumatol/klc3h6.
Nehmar R, Fauconnier L, Alves-Filho J, Togbe D, De Cauwer A, Bahram S, Le Bert M, Ryffel B, Georgel P (2021). Aryl hydrocarbon receptor (Ahr)-dependent Il-22 expression by type 3 innate lymphoid cells (ILC3) control of acute joint inflammation. J Cell Mol Med 25(10):4721-4731. doi: 10.1111/jcmm.16433.
Clere-Jehl R, Merdji H, Kassem M, Macquin C, De Cauwer A, Sibony A, Kurihara K, Minniti L, Abou Fayçal C, Bahram S, Meziani F, Helms J, Georgel P (2021). A Translational Investigation of IFN-α and STAT1 Signaling in Endothelial Cells during Septic Shock Provides Therapeutic Perspectives. Am J Respir Cell Mol Biol 65(2):167-175. doi: 10.1165/rcmb.2020-0401OC.
Lévy D, Mariotte A, DeCauwer A, Macquin C, Pichot A, Molitor A, Maurier F, Meyer A, Carapito R, Georgel P (2021). Contrasting role of NLRP12 in autoinflammation: evidence from a case report and mouse models. RMD Open 7(3):e001824. doi: 10.1136/rmdopen-2021-001824.
Georgel P (2021). Crosstalk between Interleukin-1β and Type I Interferons Signaling in Autoinflammatory Diseases. Cells10(5):1134. doi: 10.3390/cells10051134.
Georgel PT, Georgel P (2021). Where Epigenetics Meets Food Intake: Their Interaction in the Development/Severity of Gout and Therapeutic Perspectives. Front Immunol 12:752359. doi: 10.3389/fimmu.2021.752359.
Bonam SR, Areti A, Komirishetty P, Muller S (2020). Dendrimers in immunotherapy and hormone therapy. In Pharmaceutical applications of dendrimers (Eds: Chauhan A, Kulhari H), chapter 10 pages 233-249. doi: 10.1016/B978-0-12-814527-2.00010-X.
Bonam SR, Muller S (2020). Parkinson’s disease is an autoimmune disease: a reappraisal. Autoimmun Rev 19(12):102684. doi: 10.1016/j.autrev.2020.102684.
Wang F, Tasset I, Cuervo AM, Muller S (2020). In vivo remodeling of altered autophagy-lysosomal pathway by a phosphopeptide in lupus. Cells 9(10):2328. doi: 10.3390/cells9102328.
Narasaraju T, Tang BM, Herrmann M, Muller S, Chow VTK, Radic M (2020). Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front Pharmacol 11:870. doi: 10.3389/fphar.2020.00870.
Bonam SR, Muller S, Bayry J, Klionsky DJ (2020). Autophagy as an emerging target for COVID-19: lessons from an old friend, chloroquine. Autophagy 16(12):2260-2266. doi: 10.1080/15548627.2020.1779467.
Bonam SR, Tschan MP, Bayry J, Muller S (2020). Progress and challenges in the use of MAP1LC3 as a legitimate marker for measuring dynamic autophagy in vivo. Cells 9(5):1321. doi: 10.3390/cells9051321.
Voynova E, Lefebvre F, Qadri A, Muller S (2020). Correction of autophagy impairment inhibits pathology in the NOD.H-2h4 mouse model of primary Sjögren's syndrome. J Autoimmun 108:102418. doi: 10.1016/j.jaut.2020.102418.
Mariotte A, De Cauwer A, Po C, Abou-Faycal C, Pichot A, Paul N, Aouadi I, Carapito R, Frisch B, Macquin C, Chatelus E, Sibilia J, Armspach JP, Bahram S, Georgel P (2020). A mouse model of MSU-induced acute inflammation in vivo suggests imiquimod-dependent targeting of Il-1β as relevant therapy for gout patients. Theranostics 10(5):2158-2171. doi: 10.7150/thno.40650.
Kuchler-Bopp S*, Mariotte A, Strub M, Po C, De Cauwer A, Schulz G, Van Bellinghen X, Fioretti F, Clauss F, Georgel P*, Benkirane-Jessel N, Bornert F (2020). Temporomandibular joint damage in K/BxN arthritic mice. Int J Oral Sci 12(1):5. doi: 10.1038/s41368-019-0072-z.
Clere-Jehl R, Mariotte A, Meziani F, Bahram S, Georgel P*, Helms J* (2020). JAK-STAT Targeting Offers Novel Therapeutic Opportunities in Sepsis. Trends Mol Med 26(11):987-1002. doi: 10.1016/j.molmed.2020.06.007.
Bonam SR, Wang F, Muller S (2019). Lysosomes as a therapeutic target. Nat Rev Drug Discovery 18:923-948. doi: 10.1038/s41573-019-0036-1.
Bonam SR, Ruff M, Muller S (2019). HSPA8/HSC70 in Immune Disorders: A Molecular Rheostat that Adjusts Chaperone-Mediated Autophagy Substrates. Cells 8:849. doi: 10.3390/cells8080849.
Bonam SR, Bhunia D, Muller S, Nerella SG, Alvala M, Halmuthur Mahabalarao SK (2019). Novel trisaccharide based phospholipids as immunomodulators. Int Immunopharmacol 74:105684. doi: 10.1016/j.intimp.2019.105684.
Retnakumar SV, Muller S (2019). Pharmacological Autophagy Regulators as Therapeutic Agents for Inflammatory Bowel Diseases. Trends Mol Med 25:516-537. doi: 10.1016/j.molmed.2019.03.002.
Arbogast F, Arnold J, Hammann P, Kuhn L, Chicher J, Murera D, Weishaar J, Muller S, Fauny JD, Gros F (2019). ATG5 is required for B cell polarization and presentation of particulate antigens. Autophagy 15:280-294. doi: 10.1080/15548627.2018.1516327.
Wang F, Bonam SR, Schall N, Kuhn L, Hammann P, Chaloin O, Madinier JB, Briand JP, Page N, Muller S (2018). Blocking nuclear export of HSPA8 after heat shock stress severely alters cell survival. Sci Rep 8:16820. doi: 10.1038/s41598-018-34887-6.
Bendorius M, Po C, Muller S, Jeltsch-David H (2018). From systemic inflammation to neuroinflammation: the case of neurolupus. Int J Mol Sci 19:3588. doi: 10.3390/ijms19113588.
Bendorius M, Neeli I, Wang F, Bonam SR, Dombi E, Buron N, Borgne-Sanchez A, Poulton J, Radic M, Muller S (2018). The mitochondrion-lysosome axis in adaptive and innate immunity: Effect of lupus regulator peptide P140 on mitochondria autophagy and NETosis. Front Immunol 9:2158. doi: 10.3389/fimmu.2018.02158.
Bonam SR, Wang F, Muller S (2018). Autophagy: A new concept in autoimmunity regulation and a novel therapeutic option. J Autoimmun 94:16-32. doi: 10.1016/j.jaut.2018.08.009.
Muller S (2018). Excipients: not so inert? When the excipient plays the role of an active substance, as exemplified by systemic lupus. Swiss Med Wkly 148:w14631. doi: 10.4414/smw.2018.14631.
Bonam SR, Wu YS, Tunki L, Chellian R, Kumar Halmuthur MS, Muller S, and Pandy V (2018). What Has Come out from Phytomedicines and Herbal Edibles for the Treatment of Cancer? ChemMedChem 13:1854-1872. doi: 10.1002/cmdc.201800343.
Brun S, Schall N, Bonam SR, Bigauta K, Mensah-Nyagana AG, de Sèze J, Muller S (2018). An autophagy-targeting peptide to treat chronic inflammatory demyelinating polyneuropathies. J Autoimmun 92:114-125. doi: 10.1016/j.jaut.2018.05.009.
Murera D, Arbogast F, Arnold J, Bouis D, Muller S, Gros F (2018). CD4 T cell autophagy is integral to memory maintenance. Sci Rep 8:5951. doi: 10.1038/s41598-018-23993-0.
Wilhelm M, Wang F, Schall N, Kleinmann JF, Faludi M, Nashi EP, Sibilia J, Martin T, Schaeffer E, Muller S (2018). Lupus regulator peptide P140 represses B-cell antigen differentiation by reducing HLA class II overexpression. Arthritis Rheumatol 70:1077-1088. doi: 10.1002/art.40470.
Bermúdez M, Arévalo-Pinzón G, Rubio L, Chaloin O, Muller S, Curtidor H, Patarroyo MA (2018). Receptor-ligand and parasite protein-protein interactions in Plasmodium vivax: Analysing rhoptry neck proteins 2 and 4. Cell Microbiol 20(7):e12835. doi: 10.1111/cmi.12835.
Li B, Wang F, Schall N, Muller S (2018). Rescue of autophagy and lysosome defects in salivary glands of MRL/lpr mice by a therapeutic phosphopeptide. J Autoimmun 90:132-145. doi: 10.1016/j.jaut.2018.02.005.
Kökten T, Gibot S, Lepage P, D'Alessio S, Hablot J, Ndiaye NC, Busby-Venner H, Monot C, Garnier B, Moulin D, Jouzeau JY, Hansmannel F, Danese S, Guéant JL, Muller S, Peyrin-Biroulet L (2018). TREM-1 Inhibition Restores Impaired Autophagy Activity and Reduces Colitis in Mice. J Crohns Colitis 12(2):230-244. doi: 10.1093/ecco-jcc/jjx129.

